AZELASTINE HYDROCHLORIDE
PRODUCT IDENTIFICATION
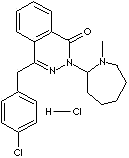
H.S. CODE
TOXICITY
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
Stable under ordinary conditions
GENERAL DESCRIPTION & APPLICATIONS
Azelastine is an antihistamine for the biological ant-allergic action to cause the symptoms of the hypersensitive and in treatment of hay fever. It belongs to H1 receptor antagonists which block the H1 type of receptors, which are involved in allergic reactions. Azelastine is used to treat allergic symptoms that may underlie the chronic inflammation as an anti-asthmatic agent. Azelastine is used intranasally as the hydrochloride salt form, white crystalline powder; sparingly soluble in water, methanol, and propylene glycol and slightly soluble in ethanol, octanol, and glycerine.
APPEARANCE
ASSAY
98.5% min